The Periodic Table is an essential map in the chemistry world, which has arranged all the basic substances in nature. This table shows not only a list of chemical elements, but also a glimpse of their characteristics and interconnection. It serves as a basic tool for chemists, physicists, and biologists, which opens the way for new discoveries. Looking at the Periodic Table, we understand how the elements are different from each other and how they combine and create everything around us. It is a living document that is constantly developing with new discoveries.
Do you know how much it has changed since the first phase table has been made? When Dmitry Mendeleev made his table, only 63 elements were known. He had some empty space, accurately assumed the characteristics of the unknown element, which was later discovered. This is really a great example of scientists’ foresight. There are currently 118 recognized elements in the modern Periodic Table, of which both natural and artificial elements exist. It is arranged according to the atomic number of the elements, which accurately reflect their electron structure and chemical behavior.
Birth of Stage Table: Mendeleev’s Vision – Periodic Table Fun Facts
The concept of the Periodic Table has been developed for many centuries, but the modern form of its modern form laid the foundation for Russian scientist Dmitry Mendeleev. He arranged the elements according to their atomic mass and noticed that the chemical properties of the elements were repeated at a certain interval. His invention was a breakthrough, because it not only classified the familiar elements, but was still able to predict the existence of some non-existent elements and their possible characteristics. Mendeleev was so confident that in some cases he believed in his periodic rules more than the known nuclear mass and arranged the elements accordingly.
Mendeleev’s table had some flaws, as some of the elements were sorted according to the atomic mass of some of their chemical properties. In 1913, a scientist named Henry Moseley used X-ray spectra and proved that the elements were found in the atomic number instead of atomic mass, and the accuracy of the atomic number was found. This discovery of Moseley gives rise to modern Periodic Tables, which are currently equipped with the growing atomic number of elements. It eliminates the defects of the table made by Mendeleev and highlights the periodic behavior of the element more clearly.
Multi-element from Rare to Rare: Phase Tables Around Us – Periodic Table Fun Facts
The Periodic Table has its own story and they play an essential role in our daily life. For example, hydrogen is the lightest and most abundant element in the universe, which helps in the formation of stars and galaxies. Oxygen, on the other hand, is essential for our breathing and a major component of water. Carbon is the basis of life, as it is the key to all organic compounds.
Interestingly, about 80% of the Periodic Table is metal. These metals include iron, an important part of hemoglobin in our blood; aluminum, which is used for lightweight construction; and gold, known for its beauty and value. Again, some elements are extremely rare and are artificially created in the laboratory, such as Tennessine or Oganesson, which are not naturally found on Earth. Each element enriches our world with its unique characteristics.
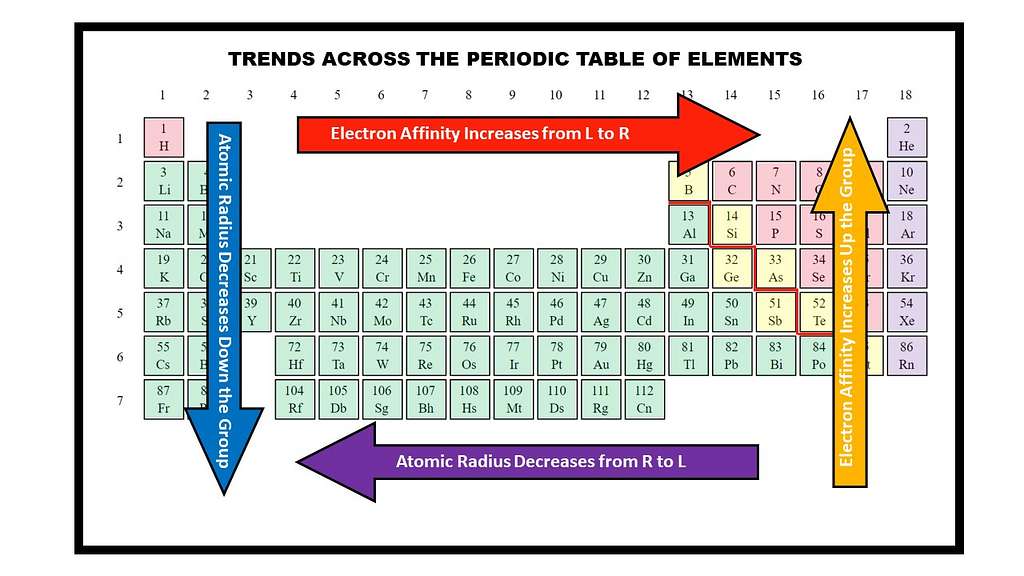
Hidden Geometry and Trends – Periodic Table Fun Facts:
The Periodic Table is not just a list; it shows the geometric format and periodic trends hidden in the physical and chemical properties of the elements. When the table goes from the left to the right and down from the top to the bottom, some of the elements’ properties regularly change. For example, the atomic size goes down in a group (vertical rows), as new electron shells are added. But at one period (horizontal rows), it is usually reduced from left to right, as the electrons are more attracted to the nucleus as the positive charges of the nucleus increase.
Similarly, features such as electronegativity and ionization energy also show specific tendencies in the Periodic Table. These trends are very important for chemists, as they can predict the chemical behavior and reactions of different elements. This mathematical and physical structure of the Periodic Table has turned it into a powerful prediction tool of chemistry, which helps to create new compounds and assume their properties.
Some Strange and Interesting Elements – Periodic Table Fun Facts
There are some elements in the Periodic Table whose features are really strange and attractive. For example, gallium is a metal that melts in the warmth of your hand, but is solid at normal room temperature. It is a popular element in scientific exhibitions. Again, mercury is the only metal that is liquid at room temperature and was used in thermometers and barometers, though its use has now decreased due to toxicity.
Silicon, which is largely found in the Earth’s crust, is the basis of the modern electronics industry. Computer chips and solar panels are made with silicon. Helium, on the other hand, is a noble gas that is used to fill airships and balloons, but it never does chemical reactions with any other element. Numerous interesting facts make each element’s story more attractive, which makes the Periodic Table alive without just being a list.
Phase Table: Future – Periodic Table Fun Facts
The Periodic Table not only holds the past and present elements, it is also a guide for future chemical discoveries. Scientists are still trying to synthesize new, superheavy elements, which exceed the existing limit of the table. These new elements are very unstable and exist for only a few milliseconds, but their discovery gives deep knowledge of our atoms and the basic forces of the universe.
Conclusion :
The Periodic Table is a living research field for scientists, where new elements are also researched, as well as the new features and applications of existing elements. It is an essential part of chemistry education and gives students a clear idea about basic physics and chemical behavior. The Periodic Table proves that science is constantly evolving and the interest of knowing the unknown is encouraging people to uncover new horizons.
The Periodic Table, far from being a mere static chart, is a dynamic and evolving “chemistry map” that has profoundly shaped our understanding of the universe. From Mendeleev’s visionary predictions to Moseley’s crucial refinement, it has consistently provided a framework for classifying elements, predicting their properties, and guiding scientific exploration. This remarkable tool not only reveals the fundamental building blocks of matter and their fascinating interconnections, but also highlights hidden geometric patterns and predictable trends that are indispensable for chemists. As a living document, the Periodic Table continues to inspire new discoveries, from the synthesis of ephemeral superheavy elements to the development of new applications for existing ones, truly demonstrating the ever-expanding frontiers of scientific inquiry.